Introduction: Patients with acute myeloid leukemia (AML) evolving from myeloproliferative neoplasms (MPN) have poor survival with median of 4-6 months and limited therapeutic options that could induce up to 60% of overall responses, but they are usually transient. Cladribine added to a backbone regimen of high dose cytarabine [≥ 1 gram per m2 intravenously (IV) for up to 5 days] and idarubicin (CLIA; further HD-clad) or low dose cytarabine [LDAC; 20 mg twice daily subcutaneously (SQ) for up to 10 days] alternating every two cycles with hypomethylating agent (HMA; regimen referred as LD-clad) have been routinely used in the treatment of AML at our institution.
Objectives: We sought to investigate the efficacy and outcome of using HD-clad or LD-clad in patients with AML arising from MPN at our center.
Methods: This is a retrospective chart review of patients with AML after previous MPN who were treated with clad-based regimen at our center and had comprehensive clinical data, including next generation molecular sequencing at AML diagnosis (≥ 28-gene myeloid panel). Since HMA-based therapy is often utilized early in MPN patients with accelerated (blasts up to 20%) or blast phase (blasts ≥20 %), and since HMA-treatment can affect outcomes, we separately evaluated patient's outcomes based on their prior exposure to HMA. Responses were according to the 2007 ELN criteria for AML and overall survival was measured from therapy initiation for AML (Kaplan-Meier method with log-rank test).
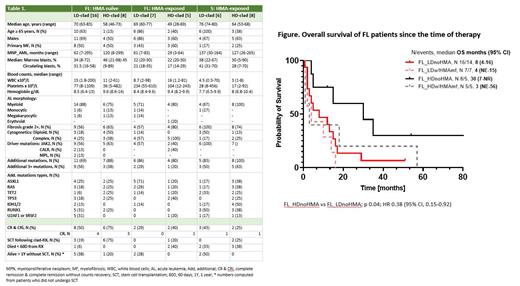
Results: The cohort includes 50 patients (median age 68 years, range 28-83; 66% males); 72%, 18%, and 10% received clad-based therapy as induction (frontline, FL), salvage 1 and salvage 2+, respectively. Among the 36 FL patients, 23 (64%) received LD-clad and 13 (36%) HD-clad regimen. 30% and 38% of patients treated with FL LD-clad and FL HD-clad were previously exposed to HMA therapy; the remaining patients were HMA-naïve. All patients in the salvage (S) setting were pre-treated with HMA. Patients' characteristics at the time of AML therapy initiation are depicted in Table 1. Median time between the diagnosis of MPN and clad-based AML therapy was 62 months (range, 1-299) for FL and 137 months (range, 26-263) for S settings. 64% of patients on FL therapy had advanced grade of reticulin fibrosis, 44% had complex cytogenetics and 56% had JAK2 mutation. Additional co-mutations were detected in 78% of FL patients, the most common (in descending order) being ASXL1, RAS, RUNX1 and TP53. Patients treated with FL HD-clad were significantly younger (median age of 61 vs 72 years for patients on FL LD-clad, p = 0.03, respectively). LD-clad was more common as FL therapy, and was used in 67% of HMA-naïve and 58% of HMA-exposed patients. The complete remission rate with and without count recovery (CR & CRi) for FL HD-clad was 62%: 75% in HMA naïve and 40% in HMA-exposed patients. CR/CRi for FL LD-clad was 43%; 50% in HMA naïve and 29% in HMA-exposed patients. 75% and 50% of patients without previous HMA-exposure were able to proceed with allogeneic stem cell transplantation (SCT) after FL HD-clad and FL LD-clad, respectively. The median OS of 30 months (95% CI, 7-unreached) was the longest in HMA-naïve patients treated with FL HD-clad regimen (Figure; median OS censored for SCT was 15 months, range: not estimated - 24). The median OS for salvage pts treated with LD-clad and HD-clad were 3.25 and 1.85 months, respectively. Among 38 patients without SCT, 10 (26%) lived for more than 12 months since therapy initiation, including 2 patients in the salvage setting.
Conclusion: Cladribine added to chemotherapy is an effective approach in patients with AML from MPN, either as an induction therapy for those eligible for SCT or acceptable long-term therapy for non-eligible patients. Noticeably, almost 40% of patients without SCT were alive past 12 months since the initiation of LD-clad therapy. HMA pretreated patients had lower response rates and poor survival even in the frontline setting.
Disclosures
Masarova:MorphoSys US: Membership on an entity's Board of Directors or advisory committees. Pemmaraju:Harborside Press: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; OncLive: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASCO Cancer.Net Editorial Board: Other: Leadership; Dava Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Menarini Group: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karger Publishers: Other: Licenses; Imedex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ClearView Healthcare Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASH Committee on Communications: Other: Leadership; Magdalen Medical Publishing: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Protagonist Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Curio Science: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Neopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pacylex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Aplastic Anemia & MDS International Foundation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CancerNet: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CareDx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ImmunoGen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Medscape: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; United States Department of Defense (DOD): Research Funding; Intellisphere: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; National Institute of Health/National Cancer Institute (NIH/NCI): Research Funding; Patient Power: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PeerView Institute for Medical Education: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Physician Education Resource (PER): Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Cimeio Therapeutics AG: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aptitude Health: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; HemOnc Times/Oncology Times: Other: Uncompensated; Bristol Myers Squibb Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Bose:GSK, Novartis, Karyopharm, AbbVie, Pharma Essentia, Jubilant, Morphic: Honoraria; Kartos, Telios, Ionis, Disc, Janssen, Geron: Research Funding; Incyte, BMS, CTI, Morphosys, Blueprint, Cogent, Sumitomo: Honoraria, Research Funding. Yilmaz:Daiichi-Sankyo: Research Funding; Pfizer: Research Funding. Short:Pfizer: Consultancy; AstraZeneca: Consultancy; Novartis: Consultancy; Stemline therapeutics: Research Funding; Astellas: Research Funding; Amgen: Honoraria; Takeda: Consultancy, Research Funding. Daver:Trovagene: Research Funding; Shattuck Labs: Consultancy; FATE: Research Funding; Hanmi: Research Funding; Novartis: Consultancy; Celgene: Consultancy; Glycomimetics: Research Funding; Trillium: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Syndax: Consultancy; AROG: Consultancy; Agios: Consultancy; Jazz: Consultancy; Kite, a Gilead company: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Novimmune: Research Funding; ImmunoGen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Servier: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Kronos Bio: Research Funding. Borthakur:Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Pacylex, Novartis, Cytomx, Bio Ascend:: Membership on an entity's Board of Directors or advisory committees; Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy. Garcia-Manero:Bristol Myers Squibb: Other: Medical writing support, Research Funding; Genentech: Research Funding; AbbVie: Research Funding. Bhalla:Foghorn Therapeutics Inc.: Research Funding. DiNardo:Fogham: Honoraria; Notable Labs: Honoraria; ImmuniOnc: Honoraria; AbbVie/Genentech: Honoraria; Servier: Honoraria; BMS: Honoraria; Astellas: Honoraria; Schrödinger: Consultancy; Novartis: Honoraria; Takeda: Honoraria. Kadia:GenFleet Therapeutics: Research Funding; Janssen Research and Development: Research Funding; Pulmotect, Inc.: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; AbbVie, Amgen, Inc, Ascentage Pharma Group, Astellas Pharma Global Development, Astex, AstraZeneca, BMS, Celgene, Cellenkos Inc, Cyclacel, Delta-Fly Pharma, Inc, Genentech, Inc., Genfleet, Glycomimetics, Iterion, Janssen Research and Development: Research Funding; Sanofi-Aventis: Consultancy; Genzyme: Honoraria; Celgene: Research Funding; Delta-Fly Pharma, Inc.: Research Funding; Genentech: Consultancy, Research Funding; Regeneron Pharmaceuticals: Research Funding; Cure: Speakers Bureau; Cyclacel: Research Funding; SELLAS Life Sciences Group: Research Funding; Novartis: Consultancy; Biologix, Cure, Hikma Pharmaceuticals: Speakers Bureau; Amgen, Inc.: Research Funding; Cellenkos Inc.: Research Funding; Jazz Pharmaceuticals, Pfizer, Pulmotect, Inc, Regeneron Pharmaceuticals, SELLAS Life Sciences Group: Research Funding; Liberum: Consultancy; Iterion: Research Funding; Glycomimetics: Research Funding; Ascentage Pharma Group: Research Funding; Astellas Pharma Global Development: Research Funding; AstraZeneca: Research Funding; Hikma Pharmaceuticals: Speakers Bureau; Agios: Consultancy; Daiichi Sankyo, Genentech, Inc., Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Inc, Pulmotect, Inc, Sanofi-Aventis, Servier: Consultancy; Servier: Consultancy; Pinotb-Bio: Consultancy; BMS: Consultancy, Research Funding; Astex: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal